Budeprion Xl Xl | Bupropion Hydrochloride Tablet, Film Coated, Extended Release while Breastfeeding

What is Budeprion Xl Xl | Bupropion Hydrochloride Tablet, Film Coated, Extended Release ?
Can I use Budeprion Xl Xl | Bupropion Hydrochloride Tablet, Film Coated, Extended Release while breastfeeding?

Budeprion Xl Xl | Bupropion Hydrochloride Tablet, Film Coated, Extended Release Breastfeeding Analsys
Bupropion hydrochloride while Breastfeeding
Low RiskCAS Number: 34911-55-2
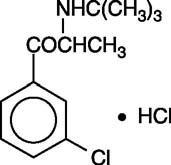
Selective inhibitor of the neuronal reuptake of catecholamines (noradrenaline and dopamine). It is used as an antidepressant and to help stop smoking (Baraona 2017). Administered orally, one daily dose. Although the concentration in milk is much higher than the plasma concentration, it is excreted in breast milk in very small amounts (Neuman 2014, Davis 2009, Haas 2004, Briggs 1993). The plasma levels of infants whose mothers were taking it were undetectable or very low (Neuman 2014, Davis 2009, Baab 2002, Briggs 1993). Given the negligible excretion in milk, the absence of plasma levels in infants and the fact that no problems were observed in infants in several publications (Nonacs 2005, Baab 2002, Briggs 1993), two cases of seizures in infants whose mothers were taking bupropion are difficult to explain, whether in monotherapy (Chaudron 2004) or associated with other antidepressants (Neuman 2014). Progress was satisfactory. Bupropion does not alter prolactin levels (Whiteman 1982). Avoid in mothers with a history of epilepsy since it decreases the seizure threshold. Until there is more published data on this drug in relation to breastfeeding, safer alternatives known may be preferable (Sriraman 2015, Carson 2013, Berle 2011, Davanzo 2011), especially during the neonatal period and in case of prematurity. See below the information of these related products:
Budeprion Xl Xl | Bupropion Hydrochloride Tablet, Film Coated, Extended Release Breastfeeding Analsys - 2
Bupropion hydrochloride while Breastfeeding
CAS Number: 34911-55-2
Limited information indicates that maternal bupropion doses of up to 300 mg daily produce low levels in breastmilk and would not be expected to cause any adverse effects in breastfed infants. However, there is little reported use in breastfed newborn infants and case reports of a possible seizure in partially breastfed 6-month-olds. If bupropion is required by a nursing mother, it is not a reason to discontinue breastfeeding. However, another drug may be preferred, especially while nursing a newborn or preterm infant. Infants exposed to bupropion and an SSRI through breastfeeding should be closely monitored for vomiting, diarrhea, jitteriness, or sedation and possibly measurement of serum levels to rule out toxicity if there is a concern.
What should I do if I am breastfeeding mother and I am already exposed to Budeprion Xl Xl | Bupropion Hydrochloride Tablet, Film Coated, Extended Release?
Budeprion Xl Xl | Bupropion Hydrochloride Tablet, Film Coated, Extended Release is in the category of low risk, if you have already used it then its not a big deal if health and behavior of baby is good. However your health care provider shall be aware of the fact that you have used Budeprion Xl Xl | Bupropion Hydrochloride Tablet, Film Coated, Extended Release so you should inform him based on your convenience.
My doctor has prescribed me Budeprion Xl Xl | Bupropion Hydrochloride Tablet, Film Coated, Extended Release, what should I do?
Budeprion Xl Xl | Bupropion Hydrochloride Tablet, Film Coated, Extended Release comes in category of low risk and if your doctor is aware that you are breastfeeding it should be ok to use
If I am using Budeprion Xl Xl | Bupropion Hydrochloride Tablet, Film Coated, Extended Release, will my baby need extra monitoring?
Not much monitoring required while using Budeprion Xl Xl | Bupropion Hydrochloride Tablet, Film Coated, Extended Release
Who can I talk to if I have questions about usage of Budeprion Xl Xl | Bupropion Hydrochloride Tablet, Film Coated, Extended Release in breastfeeding?
US
National Womens Health and Breastfeeding Helpline: 800-994-9662 (TDD 888-220-5446) 9 a.m. and 6 p.m. ET, Monday through Friday
UK
National Breastfeeding Helpline: 0300-100-0212 9.30am to 9.30pm, daily
Association of Breastfeeding Mothers: 0300-330-5453
La Leche League: 0345-120-2918
The Breastfeeding Network supporter line in Bengali and Sylheti: 0300-456-2421
National Childbirth Trust (NCT): 0300-330-0700
Australia
National Breastfeeding Helpline: 1800-686-268 24 hours a day, 7 days a week
Canada
Telehealth Ontario for breastfeeding: 1-866-797-0000 24 hours a day, 7 days a week
