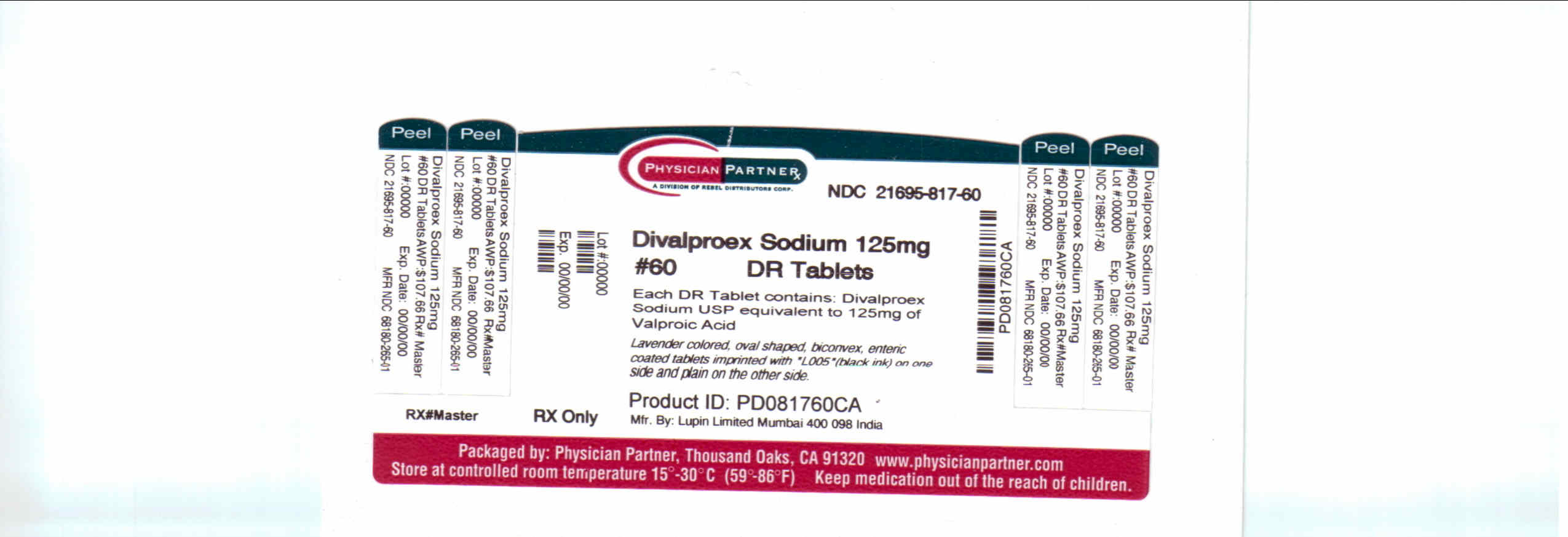
Divalproex Sodium Dr | Divalproex Sodium Tablet, Delayed Release while Breastfeeding
What is Divalproex Sodium Dr | Divalproex Sodium Tablet, Delayed Release used for?
Can I continue breastfeeding if I am using Divalproex Sodium Dr | Divalproex Sodium Tablet, Delayed Release? How long does it stays in breast milk?

Nursing Mothers Valproate is excreted in breast milk. Concentrations in breast milk have been reported to be 1- 10% of serum concentrations. It is not known what effect this would have on a nursing infant. Consideration should be given to discontinuing nursing when divalproe sodium is administered to a nursing woman.
Divalproex Sodium Dr | Divalproex Sodium Tablet, Delayed Release Breastfeeding Analsys
Valproic acid while Breastfeeding
SafeCAS Number: 99-66-1
It is excreted in breast milk in clinically non-significant amount without problems in the short or long term in infants whose mothers were treated. Plasma levels of these infants were undetectable or very low. Cognitive development did not suffer any alteration. An infant was presented with thrombocytopenic purpura and anemia who recovered after removing valproate in the mother. However, doubts were raised on whether it was instead a post-viral reaction that led to idiopathic thrombocytopenic purpura. The administration of valproic acid does not affect prolactin levels. The American Academy of Pediatrics rates it as usual compatible with breastfeeding medication.WHO List of Essential Medicines 2002: compatible with breastfeeding.
Divalproex Sodium Dr | Divalproex Sodium Tablet, Delayed Release Breastfeeding Analsys - 2
Valproic acid while Breastfeeding
CAS Number: 99-66-1
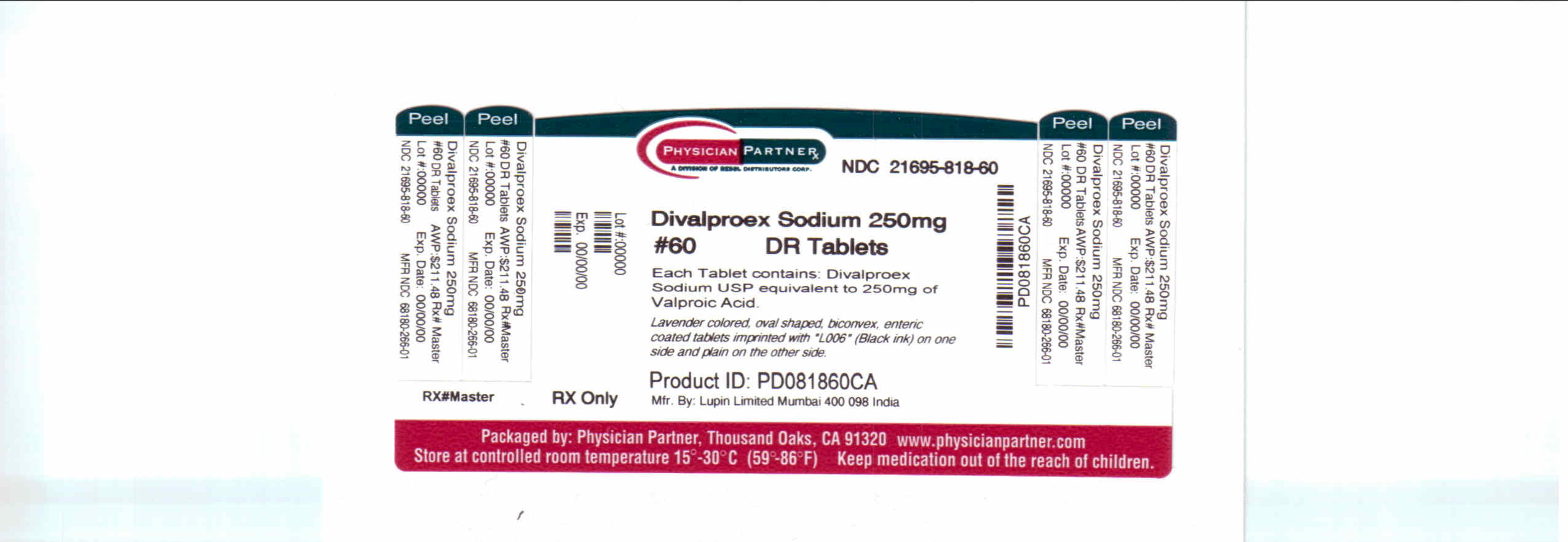
Breastfeeding during valproic acid monotherapy does not appear to adversely affect infant growth or development, and breastfed infants had higher IQs and enhanced verbal abilities than nonbreastfed infants at 6 years of age in one study.[1] If valproic acid is required by the mother, it is not necessarily a reason to discontinue breastfeeding. Because of the low levels of valproic acid in breastmilk and infant serum, no definite adverse reactions to valproic acid during breastfeeding have been reported. Theoretically, breastfed infants are at risk for valproic acid-induced hepatotoxicity, so infants should be monitored for jaundice and other signs of liver damage during maternal therapy. A questionable case of thrombocytopenia has been reported, so monitor the infant for unusual bruising or bleeding. One author recommends monitoring infant serum valproate levels, platelets and liver enzymes during therapy.[1] Combination therapy with sedating anticonvulsants or psychotropics may result in infant sedation or withdrawal reactions.

I already used Divalproex Sodium Dr | Divalproex Sodium Tablet, Delayed Release and meanwhile I breastfed my baby should I be concerned?
It is always a good idea to keep your healthcare provider or doctor informed about your drug usage during pregnancy and breastfeeding but if you have not informed your doctor about Divalproex Sodium Dr | Divalproex Sodium Tablet, Delayed Release and have used it then do not panic as Divalproex Sodium Dr | Divalproex Sodium Tablet, Delayed Release is mostly safe in breastfeeding and should not cause any harm to your baby.
My doctor has prescribed me Divalproex Sodium Dr | Divalproex Sodium Tablet, Delayed Release, what should I do?
Definitely, Divalproex Sodium Dr | Divalproex Sodium Tablet, Delayed Release is safe in lactation for baby. No wonder your doctor has recommended it.
If I am using Divalproex Sodium Dr | Divalproex Sodium Tablet, Delayed Release, will my baby need extra monitoring?
No extra baby monitoring required while mother is using Divalproex Sodium Dr | Divalproex Sodium Tablet, Delayed Release
Who can I talk to if I have questions about usage of Divalproex Sodium Dr | Divalproex Sodium Tablet, Delayed Release in breastfeeding?
US
National Womens Health and Breastfeeding Helpline: 800-994-9662 (TDD 888-220-5446) 9 a.m. and 6 p.m. ET, Monday through Friday
UK
National Breastfeeding Helpline: 0300-100-0212 9.30am to 9.30pm, daily
Association of Breastfeeding Mothers: 0300-330-5453
La Leche League: 0345-120-2918
The Breastfeeding Network supporter line in Bengali and Sylheti: 0300-456-2421
National Childbirth Trust (NCT): 0300-330-0700
Australia
National Breastfeeding Helpline: 1800-686-268 24 hours a day, 7 days a week
Canada
Telehealth Ontario for breastfeeding: 1-866-797-0000 24 hours a day, 7 days a week
