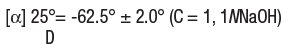It is recommended to breastfeed exclusively for six months and then while introducing to other food sources extend it to twelve months. In this duration most mothers will need help of some sort of medication, It could be for short term like could and flue or it could be something chronic like Arthritis or Diabetes and here comes the question of safety of medication in use. In this post we will figure out what is Depen | Penicillamine Tablet and whether its safe to use Depen | Penicillamine Tablet while nursing or not.
What is Depen | Penicillamine Tablet used for?
- DEPEN is indicated in the treatment of Wilson’s disease, cystinuria, and in patients with severe, active rheumatoid arthritis who have failed to respond to an adequate trial of conventional therapy. Available evidence suggests that DEPEN is not of value in ankylosing spondylitis. Wilson’s Disease - Wilson’s disease (hepatolenticular degeneration) results from the interaction of an inherited defect and an abnormality in copper metabolism. The metabolic defect, which is the consequence of the autosomal inheritance of one abnormal gene from each parent, manifests itself in a greater positive copper balance than normal. As a result, copper is deposited in several organs and appears eventually to produce pathologic effects most prominently seen in the brain, where degeneration is widespread; in the liver, where fatty infiltration, inflammation, and hepatocellular damage progress to postnecrotic cirrhosis; in the kidney, where tubular and glomerular dysfunction results; and in the eye, where characteristic corneal copper deposits are known as Kayser-Fleischer rings. Two types of patients require treatment for Wilson’s disease: (1) the symptomatic, and (2) the asymptomatic in whom it can be assumed the disease will develop in the future if the patient is not treated. Diagnosis, suspected on the basis of family or individual history, physical examination, or a low serum concentration of ceruloplasmin*, is confirmed by the demonstration of Kayser-Fleischer rings or, particularly in the asymptomatic patient, by the quantitative demonstration in a liver biopsy specimen of a concentration of copper in excess of 250 mcg/g dry weight. Treatment has two objectives: (1) to minimize dietary intake and absorption of copper. (2) to promote excretion of copper deposited in tissues. The first objective is attained by a daily diet that contains no more than one or two milligrams of copper. Such a diet should exclude, most importantly, chocolate, nuts, shellfish, mushrooms, liver, molasses, broccoli, and cereals enriched with copper, and be composed to as great an extent as possible of foods with a low copper content. Distilled or demineralized water should be used if the patient’s drinking water contains more than 0.1 mg of copper per liter. For the second objective, a copper chelating agent is used. In symptomatic patients, this treatment usually produces marked neurologic improvement, fading of Kayser-Fleischer rings, and gradual amelioration of hepatic dysfunction and psychic disturbances. Clinical experience to date suggests that life is prolonged with the above regimen. Noticeable improvement may not occur for one to three months. Occasionally, neurologic symptoms become worse during initiation of therapy with DEPEN. Despite this, the drug should not be discontinued permanently. Although temporary interruption may result in clinical improvement of the neurological symptoms, it carries an increased risk of developing a sensitivity reaction upon resumption of therapy (See WARNINGS). * For quantitative test for serum ceruloplasmin see: Morell, A.G.; Windsor, J.; Sternlieb, I; Scheinberg, I.H.: Measurement of the concentration of ceruloplasmin in serum by determination of its oxidase activity, in “Laboratory Diagnosis of Liver Disease,” F.W. Sunderman; F.W. Sunderman, Jr., (eds.), St. Louis, Warren H. Green, Inc., 1968, pp. 193-195. Treatment of asymptomatic patients has been carried out for over ten years. Symptoms and signs of the disease appear to be prevented indefinitely if daily treatment with DEPEN can be continued. Cystinuria - Cystinuria is characterized by excessive urinary excretion of the dibasic amino acids, arginine, lysine, ornithine, and cystine, and the mixed disulfide of cysteine and homocysteine. The metabolic defect that leads to cystinuria is inherited as an autosomal, recessive trait. Metabolism of the affected amino acids is influenced by at least two abnormal factors: (1) defective gastrointestinal absorption and (2) renal tubular dysfunction. Arginine, lysine, ornithine, and cysteine are soluble substances, readily excreted. There is no apparent pathology connected with their excretion in excessive quantities. Cystine, however, is so slightly soluble at the usual range of urinary pH that it is not excreted readily, and so crystallizes and forms stones in the urinary tract. Stone formation is the only known pathology in cystinuria. Normal daily output of cystine is 40 to 80 mg. In cystinuria, output is greatly increased and may exceed 1 g/day. At 500 to 600 mg/day, stone formation is almost certain. When it is more than 300 mg/day, treatment is indicated. Conventional treatment is directed at keeping urinary cystine diluted enough to prevent stone formation, keeping the urine alkaline enough to dissolve as much cystine as possible, and minimizing cystine production by a diet low in methionine (the major dietary precursor of cystine). Patients must drink enough fluid to keep urine specific gravity below 1.010, take enough alkali to keep urinary pH at 7.5 to 8, and maintain a diet low in methionine. This diet is not recommended in growing children and probably is contraindicated in pregnancy because of its low protein content (see PRECAUTIONS). When these measures are inadequate to control recurrent stone formation, DEPEN may be used as additional therapy. When patients refuse to adhere to conventional treatment, DEPEN may be a useful substitute. It is capable of keeping cystine excretion to near normal values, thereby hindering stone formation and the serious consequences of pyelonephritis and impaired renal function that develop in some patients. Bartter and colleagues depict the process by which penicillamine interacts with cystine to form penicillamine-cysteine mixed disulfide as: In this process, it is assumed that the deprotonated form of penicillamine, PS', is the active factor in bringing about the disulfide interchange. Rheumatoid Arthritis - Because DEPEN can cause severe adverse reactions, its use in rheumatoid arthritis should be restricted to patients who have severe, active disease and who have failed to respond to an adequate trial of conventional therapy. Even then, benefit-to-risk ratio should be carefully considered. Other measures, such as rest, physiotherapy, salicylates, and corticosteroids should be used, when indicated, in conjunction with DEPEN (see PRECAUTIONS). Drug Configuration
I am breastfeeding mother and I am using Depen | Penicillamine Tablet. Can it have any bad effect on my kid? Shall I search for better alternative?
Penicillamine is the one and only active ingredient present in Depen | Penicillamine Tablet. Penicillamine in itself is a low risk drug for lactation so it is easy to understand that Depen | Penicillamine Tablet also comes in category of Low Risk item while breastfeeding. Below is the summary of Penicillamine in breastfeeding.
Statement of Manufacturer/Labeler about breastfeeding usage
Nursing Mothers - See CONTRAINDICATIONS.
Depen | Penicillamine Tablet Breastfeeding Analsys
Low RiskCAS Number: 52-66-4
A chelating agent that helps remove heavy metals such as copper, lead and mercury from the body.Used to treat Wilson's disease, cystinuria, severe rheumatoid arthritis and chronic active hepatitis. It is excreted in breast milk in clinically insignificant amounts (undetectable levels: Izumi 2012) and no problems have been observed in infants whose mothers have taken it (Sternlieb 2000, Messner 1998, Gregory 1983). Oral bioavailability decreases by half in the presence of food, so its passing to the infant’s plasma via ingested breast milk would be very difficult Copper and zinc levels in breast milk of mothers treated with penicillamine, trientine or zinc are normal according to more recent studies (Izumi 2012) and lower than normal according to older ones (Bunke 1989). Cases of breast hyperplasia and hyperprolactinemia have been reported in patients treated with penicillamine (Craig 1988, Kahl 1985, Thew 1980).
Depen | Penicillamine Tablet Breastfeeding Analsys - 2
CAS Number: 52-67-5
Limited information indicates that penicillamine is not detectable in breastmilk. Copper and zinc levels in breastmilk are reduced in mothers receiving penicillamine.[1][2][3][4][5] Penicillamine has been used with apparent safety during nursing of 3 infants. In infants who breastfeed infrequently, taking the drug right after nursing and waiting 4 to 6 hours before nursing again should minimize the amount of penicillamine in breastmilk. Copper and zinc levels in breastmilk are reduced in patients taking penicillamine. The implications for infants of this effect are not known.
What if I already have used Depen | Penicillamine Tablet?
Depen | Penicillamine Tablet is in the category of low risk, if you have already used it then its not a big deal if health and behavior of baby is good. However your health care provider shall be aware of the fact that you have used Depen | Penicillamine Tablet so you should inform him based on your convenience.
I am nursing mother and my doctor has suggested me to use Depen | Penicillamine Tablet, is it safe?
Depen | Penicillamine Tablet comes in category of low risk and if your doctor is aware that you are breastfeeding it should be ok to use without much concerns.
If I am using Depen | Penicillamine Tablet, will my baby need extra monitoring?
Not much monitoring required while using Depen | Penicillamine Tablet
Who can I talk to if I have questions about usage of Depen | Penicillamine Tablet in breastfeeding?
US
National Womens Health and Breastfeeding Helpline: 800-994-9662 (TDD 888-220-5446) 9 a.m. and 6 p.m. ET, Monday through Friday
UK
National Breastfeeding Helpline: 0300-100-0212 9.30am to 9.30pm, daily
Association of Breastfeeding Mothers: 0300-330-5453
La Leche League: 0345-120-2918
The Breastfeeding Network supporter line in Bengali and Sylheti: 0300-456-2421
National Childbirth Trust (NCT): 0300-330-0700
Australia
National Breastfeeding Helpline: 1800-686-268 24 hours a day, 7 days a week
Canada
Telehealth Ontario for breastfeeding: 1-866-797-0000 24 hours a day, 7 days a week