Actisep | Benzocaine, Menthol, Cetylpyridinium Chloride Solution while Breastfeeding
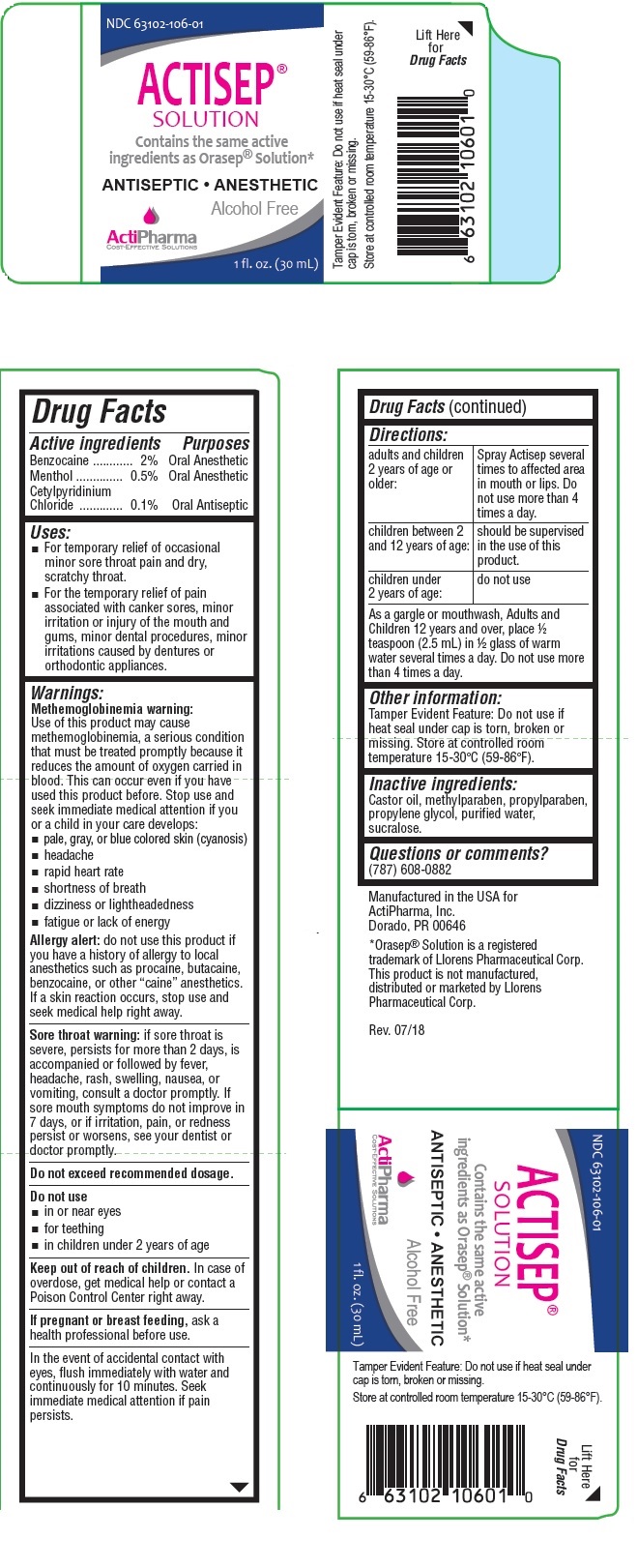
What is Actisep | Benzocaine, Menthol, Cetylpyridinium Chloride Solution used for?
Brief: Oral Anesthetic Oral Anesthetic Oral Antiseptic
Can I continue breastfeeding if I am using Actisep | Benzocaine, Menthol, Cetylpyridinium Chloride Solution? How long does it stays in breast milk?
Actisep | Benzocaine, Menthol, Cetylpyridinium Chloride Solution Breastfeeding Analsys
Benzocaine while Breastfeeding
SafeCAS Number: 94-09-7
At latest update, relevant published data on excretion into breast milk were not found. Topical anesthetics (intended for dermatological or oral use) when properly used, show limited systemic absorption which is practically nil, with nil or non-significant systemic levels in the plasma and breast milk. Benzocaine may induce appearance of methemoglobinemia and systemic toxicity if absorbed. Avoid use on the breast, otherwise, in case of use on the nipple, let it be done after a feed and wipe it out by thoroughly washing with water before the next feed. Do not apply creams, gels and other products that would contain paraffin (mineral oil) to avoid absorption by the infant since it is a hydrocarbon-derived substance.
Menthol, unspecified form while Breastfeeding
SafeHerb which is widely used by many cultures. It has been used even for pain relief during pregnancy and colicky pain in fussy babies (without proved data on this). Since it is non toxic at appropriate dose and a tiny excretion into breast milk of active metabolite Menthol, a moderate consumption is believed compatible while breastfeeding. Dessicated leaves and essential oil of the plant that contains Menthol are used. Properties that have been demonstrated and approved indications are: as spasmolytic for Dyspepsia, Irritable Colon and flatulence. It has been used for the treatment of cracked nipple with best results than placebo or Lanolin. Although with no proven effectiveness, it is traditionally used for cough relief, common cold, pain or itching by local application or inhalation. Overdosing of essential oil may be harmful. Do not expose infants to inhalation of products that contain Menthol (irritation of the air way) In case of use on the nipple, do it after feeding the baby and cleanse thoroughly the surface before the next one.
Actisep | Benzocaine, Menthol, Cetylpyridinium Chloride Solution Breastfeeding Analsys - 2
Benzocaine while Breastfeeding
CAS Number: 94-09-7
Topical benzocaine has not been studied during breastfeeding, but is unlikely to affect her breastfed infant if it is applied away from the breast. Benzocaine should not be applied to the breast or nipple, because the infant may ingest the drug during nursing and it has been associated with severe methemoglobinemia.
Menthol, unspecified form while Breastfeeding
Peppermint (Mentha x piperita) contains menthol, menthone, menthyl acetate as major ingredients. Minor ingredients include 1,8-cineole, pulegone, bitter substances, caffeic acid, flavonoids, and tannins. Peppermint is a purported galactogogue; however, no scientifically valid clinical trials support this use.[1] Galactogogues should never replace evaluation and counseling on modifiable factors that affect milk production.[2] Topical peppermint gel and solutions have been studied for the prevention of pain and cracked nipples and areolas in nursing women. The peppermint preparations were more effective than placebo and expressed breastmilk, and about as effective as lanolin,[3][4][5][6] although a meta-analysis concluded that application of nothing or breastmilk may be superior to lanolin, but good studies are lacking.[7] Menthol is excreted into breastmilk in small quantities; the excretion of other components have not been studied. Peppermint is "generally recognized as safe" (GRAS) as a food by the U.S. Food and Drug Administration. Large doses can cause heartburn, nausea and vomiting. Allergic reactions, including headache, have been reported to menthol. If peppermint is used on the nipples, it should be used after nursing and wiped off before the next nursing. Dietary supplements do not require extensive pre-marketing approval from the U.S. Food and Drug Administration. Manufacturers are responsible to ensure the safety, but do not need to the safety and effectiveness of dietary supplements before they are marketed. Dietary supplements may contain multiple ingredients, and differences are often found between labeled and actual ingredients or their amounts. A manufacturer may contract with an independent organization to verify the quality of a product or its ingredients, but that does certify the safety or effectiveness of a product. Because of the above issues, clinical testing results on one product may not be applicable to other products. More detailed information #about dietary supplements# is available elsewhere on the LactMed Web site.
What should I do if I am breastfeeding mother and I am already exposed to Actisep | Benzocaine, Menthol, Cetylpyridinium Chloride Solution?
Not much study has been done on safety of Actisep | Benzocaine, Menthol, Cetylpyridinium Chloride Solution in breastfeeding and its ingredients. Even we do not have complete information about usage of Actisep | Benzocaine, Menthol, Cetylpyridinium Chloride Solution in breastfeeding so at this point a trained medical professional could be your best bet. If you observe anything abnormal with your baby please contact 911.
My doctor has prescribed me Actisep | Benzocaine, Menthol, Cetylpyridinium Chloride Solution, what should I do?
If your doctor considers Actisep | Benzocaine, Menthol, Cetylpyridinium Chloride Solution safe enough to prescribe for you that means its benefits should outweigh its known risks for you.
If I am using Actisep | Benzocaine, Menthol, Cetylpyridinium Chloride Solution, will my baby need extra monitoring?
We are not Sure, Please check with your healthcare provider or doctor.
Who can I talk to if I have questions about usage of Actisep | Benzocaine, Menthol, Cetylpyridinium Chloride Solution in breastfeeding?
US
National Womens Health and Breastfeeding Helpline: 800-994-9662 (TDD 888-220-5446) 9 a.m. and 6 p.m. ET, Monday through Friday
UK
National Breastfeeding Helpline: 0300-100-0212 9.30am to 9.30pm, daily
Association of Breastfeeding Mothers: 0300-330-5453
La Leche League: 0345-120-2918
The Breastfeeding Network supporter line in Bengali and Sylheti: 0300-456-2421
National Childbirth Trust (NCT): 0300-330-0700
Australia
National Breastfeeding Helpline: 1800-686-268 24 hours a day, 7 days a week
Canada
Telehealth Ontario for breastfeeding: 1-866-797-0000 24 hours a day, 7 days a week
