Amoxicillin And Clavulanate Potassium Tablet, Multilayer, Extended Release while Breastfeeding
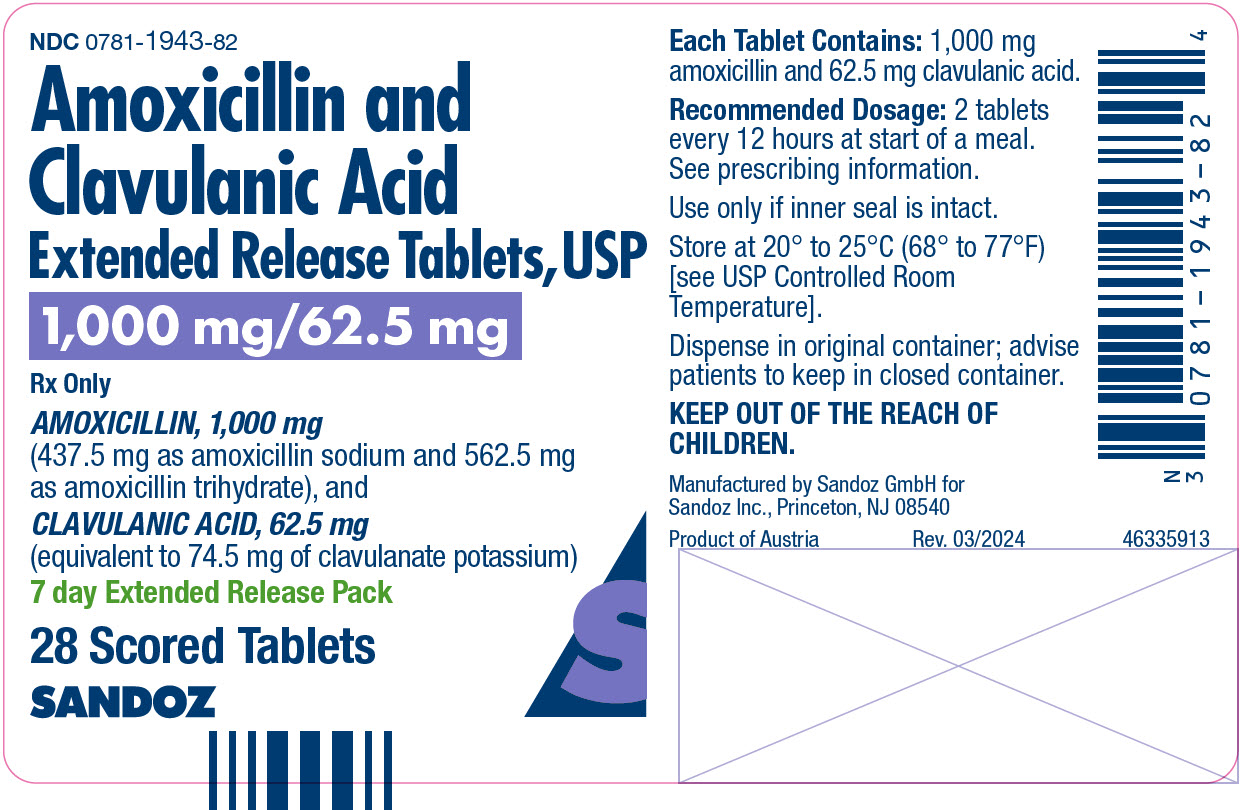
What is Amoxicillin And Clavulanate Potassium Tablet, Multilayer, Extended Release ?
Is using Amoxicillin And Clavulanate Potassium Tablet, Multilayer, Extended Release safe or dangerous while breastfeeding?

8.3 Nursing Mothers Amoxicillin has been shown to be excreted in human milk; therefore, caution should be exercised when Amoxicillin and Clavulanate Potassium Extended Release Tablets is administered to a nursing woman.
Amoxicillin And Clavulanate Potassium Tablet, Multilayer, Extended Release Breastfeeding Analsys
Amoxicillin anhydrous while Breastfeeding
SafeCAS Number: 26787-78-0
Excretion into breast milk is insignificant and no adverse effects reported in breasted infants. Es considerada medicación compatible con la lactancia por autores y sociedades científicas relevantes (Rowe 2013, Nahum 2006, Mahadevan 2006, Bar-Oz 2003, CDC 2001, Fulton 1992). Es una medicación de uso común en Pediatría y considerado medicamento esencial para uso pediátrico por la OMS (WHO 2013). The possible negativity of cultures in febrile infants whose mothers take antibiotics should be taken into account, as well as the possibility of gastroenteritis due to altered intestinal flora (Benyamini 2005, Ito 1993, Kafetzis 1981). American Academy of Pediatrics: medication usually compatible with breastfeeding (AAP 2001).List of WHO essential medicines: compatible with breastfeeding (WHO / UNICEF 2002).
Amoxicillin anhydrous while Breastfeeding
SafeCAS Number: 26787-78-0
Excretion into breast milk is insignificant and no adverse effects reported in breasted infants. Es considerada medicación compatible con la lactancia por autores y sociedades científicas relevantes (Rowe 2013, Nahum 2006, Mahadevan 2006, Bar-Oz 2003, CDC 2001, Fulton 1992). Es una medicación de uso común en Pediatría y considerado medicamento esencial para uso pediátrico por la OMS (WHO 2013). The possible negativity of cultures in febrile infants whose mothers take antibiotics should be taken into account, as well as the possibility of gastroenteritis due to altered intestinal flora (Benyamini 2005, Ito 1993, Kafetzis 1981). American Academy of Pediatrics: medication usually compatible with breastfeeding (AAP 2001).List of WHO essential medicines: compatible with breastfeeding (WHO / UNICEF 2002).
Amoxicillin And Clavulanate Potassium Tablet, Multilayer, Extended Release Breastfeeding Analsys - 2
Amoxicillin anhydrous while Breastfeeding
CAS Number: 26787-78-0
Limited information indicates that amoxicillin produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Occasionally, rash and disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush, have been reported, but these effects have not been adequately evaluated. Amoxicillin is acceptable in nursing mothers. Amoxicillin powder for suspension reconstituted with breastmilk is absorbed as well as the powder reconstituted with water.[1]
Amoxicillin anhydrous while Breastfeeding
CAS Number: 26787-78-0
Limited information indicates that amoxicillin produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Occasionally, rash and disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush, have been reported, but these effects have not been adequately evaluated. Amoxicillin is acceptable in nursing mothers. Amoxicillin powder for suspension reconstituted with breastmilk is absorbed as well as the powder reconstituted with water.[1]
Clavulanic acid while Breastfeeding
CAS Number: 86482-18-0
Limited information indicates that ticarcillin produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Clavulanic acid has not been studied in nursing mothers. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with penicillins, but these effects have not been adequately evaluated. Ticarcillin and clavulanic acid is acceptable is acceptable in nursing mothers.
Amoxicillin And Clavulanate Potassium Tablet, Multilayer, Extended Release Breastfeeding Analsys - 3
Clavulanic acid and Breastfeeding
SafeWhat should I do if already breastfed my kid after using Amoxicillin And Clavulanate Potassium Tablet, Multilayer, Extended Release?
It is always a good idea to keep your healthcare provider or doctor informed about your drug usage during pregnancy and breastfeeding but if you have not informed your doctor about Amoxicillin And Clavulanate Potassium Tablet, Multilayer, Extended Release and have used it then do not panic as Amoxicillin And Clavulanate Potassium Tablet, Multilayer, Extended Release is mostly safe in breastfeeding and should not cause any harm to your baby.
My doctor has prescribed me Amoxicillin And Clavulanate Potassium Tablet, Multilayer, Extended Release, what should I do?
Usage of Amoxicillin And Clavulanate Potassium Tablet, Multilayer, Extended Release is safe for nursing mothers and baby, No worries.
If I am using Amoxicillin And Clavulanate Potassium Tablet, Multilayer, Extended Release, will my baby need extra monitoring?
No
Who can I talk to if I have questions about usage of Amoxicillin And Clavulanate Potassium Tablet, Multilayer, Extended Release in breastfeeding?
US
National Womens Health and Breastfeeding Helpline: 800-994-9662 (TDD 888-220-5446) 9 a.m. and 6 p.m. ET, Monday through Friday
UK
National Breastfeeding Helpline: 0300-100-0212 9.30am to 9.30pm, daily
Association of Breastfeeding Mothers: 0300-330-5453
La Leche League: 0345-120-2918
The Breastfeeding Network supporter line in Bengali and Sylheti: 0300-456-2421
National Childbirth Trust (NCT): 0300-330-0700
Australia
National Breastfeeding Helpline: 1800-686-268 24 hours a day, 7 days a week
Canada
Telehealth Ontario for breastfeeding: 1-866-797-0000 24 hours a day, 7 days a week
