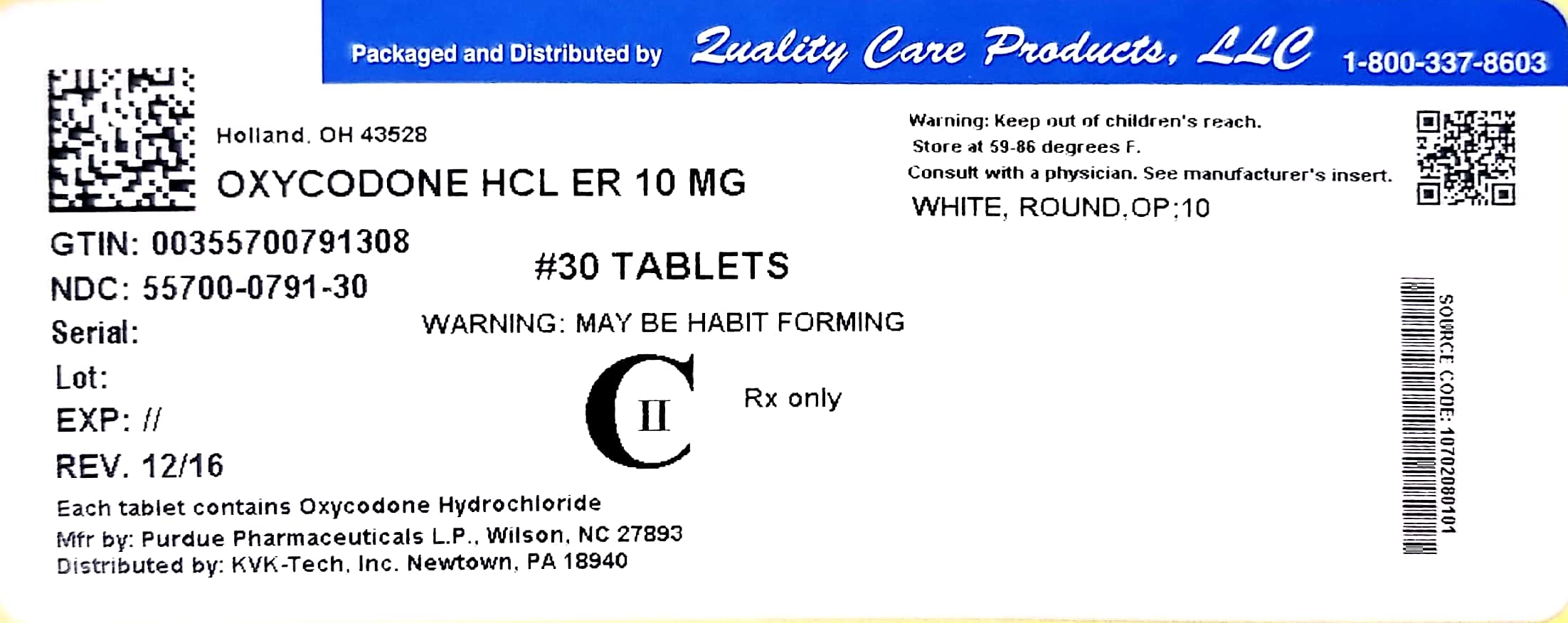
Oxycodone Hcl Tablet, Film Coated, Extended Release while Breastfeeding
What is Oxycodone Hcl Tablet, Film Coated, Extended Release used for?
Is using Oxycodone Hcl Tablet, Film Coated, Extended Release unsafe in breastfeeding? Can there be bad consequences for baby if I use it while breastfeeding?

Oxycodone Hcl Tablet, Film Coated, Extended Release Breastfeeding Analsys
Oxycodone hydrochloride while Breastfeeding
UnsafeCAS Number: 76-42-6
Very often used for treatment of pain associated to episiotomy or Cesarean section operation. Excreted and accumulates into breast milk in significant amount along with associated problems among 20% of breastfed infants from treated mothers. Side effects have been rarely severe like excessive sedation, letargia, hypothermia and apnea. Dose should not be higher than 30 mg a day for no longer than 3 days. Women with some variants of enzyme-linked gene CYP2D6 who are on Oxycodone and their breastfed infants may experience increased sedation. Dose should not be higher than 30 mg a day for no longer than 3 days. Use of Oxycodone during childhood is risky because of a large elimination half-life variability. Adequately use of nonsteroidal anti-inflammatory drugs (NSAIDs) may attain pain relief with less side effects than with narcotic analgesics.
Oxycodone Hcl Tablet, Film Coated, Extended Release Breastfeeding Analsys - 2
Oxycodone hydrochloride while Breastfeeding
CAS Number: 76-42-6
Maternal use of oral narcotics during breastfeeding can cause infant drowsiness, central nervous system depression and even death. Infant sedation is common and well documented with maternal use of oxycodone. Newborn infants seem to be particularly sensitive to the effects of even small dosages of narcotic analgesics. Once the mother's milk comes in, it is best to provide pain control with a nonnarcotic analgesic and limit maternal intake of oral oxycodone (and combinations) to a 2 to 3 days, especially in the outpatient setting.[1] A maximum oxycodone dosage of 30 mg daily is suggested, although some sources recommend avoiding oxycodone during breastfeeding.[2][3] Oxycodone elimination is decreased in young infants with much inter-individual variability. Monitor the infant closely for drowsiness, adequate weight gain, and developmental milestones, especially in younger, exclusively breastfed infants. If the baby shows signs of increased sleepiness (more than usual), difficulty breastfeeding, breathing difficulties, or limpness, a physician should be contacted immediately. Other agents are preferred over oxycodone during breastfeeding.[2]
What if I already have used Oxycodone Hcl Tablet, Film Coated, Extended Release?
If you observer abnormal behavior or any other health issue in infant then you should immediately call 911 or contact other contact other emergency service provider in your area otherwise closely monitor the baby and inform your doctor about your Oxycodone Hcl Tablet, Film Coated, Extended Release usage and time interval of breastfeeding.
My health care provider has asked me to use Oxycodone Hcl Tablet, Film Coated, Extended Release, what to do?
If your doctor knows that you are breastfeeding mother and still prescribes Oxycodone Hcl Tablet, Film Coated, Extended Release then there must be good reason for that as Oxycodone Hcl Tablet, Film Coated, Extended Release is considered unsafe, It usually happens when doctor finds that overall advantage of taking
If I am using Oxycodone Hcl Tablet, Film Coated, Extended Release, will my baby need extra monitoring?
Yes, Extra monitoring is required if mother is using Oxycodone Hcl Tablet, Film Coated, Extended Release and breastfeeding as it is considered unsafe for baby.
Who can I talk to if I have questions about usage of Oxycodone Hcl Tablet, Film Coated, Extended Release in breastfeeding?
US
National Womens Health and Breastfeeding Helpline: 800-994-9662 (TDD 888-220-5446) 9 a.m. and 6 p.m. ET, Monday through Friday
UK
National Breastfeeding Helpline: 0300-100-0212 9.30am to 9.30pm, daily
Association of Breastfeeding Mothers: 0300-330-5453
La Leche League: 0345-120-2918
The Breastfeeding Network supporter line in Bengali and Sylheti: 0300-456-2421
National Childbirth Trust (NCT): 0300-330-0700
Australia
National Breastfeeding Helpline: 1800-686-268 24 hours a day, 7 days a week
Canada
Telehealth Ontario for breastfeeding: 1-866-797-0000 24 hours a day, 7 days a week
