Visible Difference Multi Targeted Bb Cream Broad Spectrum Sunscreen Spf 30 Shade 1 while Breastfeeding
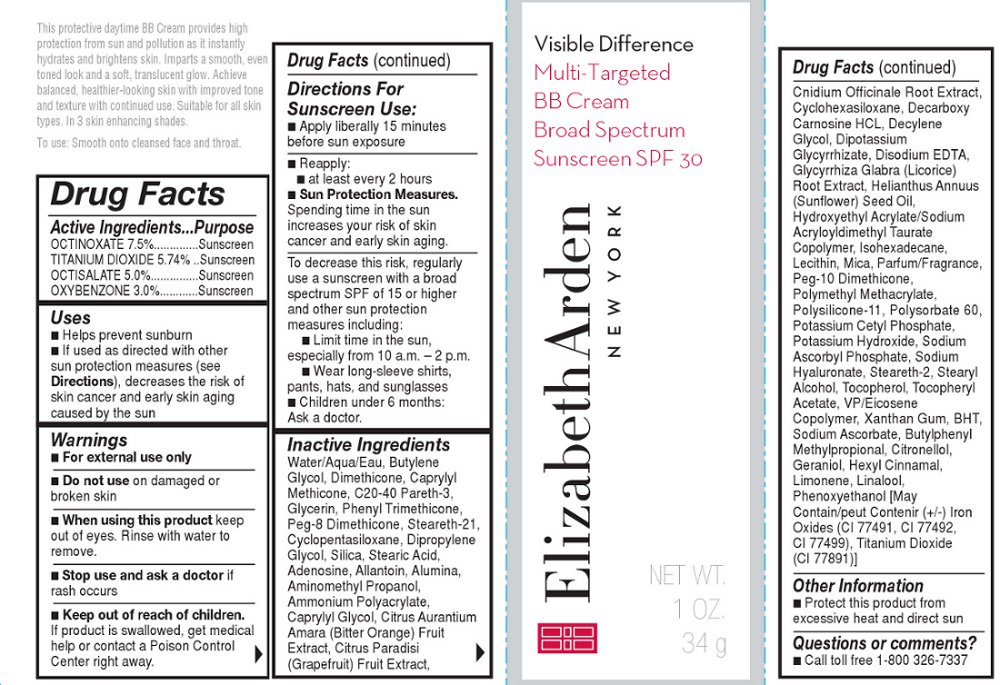
What is Visible Difference Multi Targeted Bb Cream Broad Spectrum Sunscreen Spf 30 Shade 1 ?
Brief: OTC - PURPOSE Provides SPF 30 sun protection
Can I use Visible Difference Multi Targeted Bb Cream Broad Spectrum Sunscreen Spf 30 Shade 1 while breastfeeding?

Octinoxate and Breastfeeding
UnsafeOctinoxate (Octylmethoxycinnamate) has been detected in human urine, blood and breast milk and is known for moderate risk of skin allergy. Some studies suggest that Octinoxate has estrogen like effects however less than 1% skin penetration has been found in human laboratory studies. As not much study has been done on effects of Octinoxate during breast feeding its recommended to use safe alternatives.
Octyl Methoxycinnamate (OMC) is a frequently used UV-filter in sunscreens and other cosmetics. Octinoxate can be systemically absorbed after skin application, being found in the deeper layers of the stratum corneum as well as urine, plasma, and breast milk. The mean maximum plasma concentration detected after application of 2mg/cm2 sunscreen was 7ng/mL in women and 16ng/mL in men. FDA study found blood levels 13 times above cutoff for systemic exposure.
Several studies indicated that OMC acts as an endocrine disruptor due to the ability to interfere with endocrine system at different levels. In humans OMC exposure has minor, but statistically significant effects on the levels of testosterone and estradiol. Moreover, some studies suggested that OMC can interact with the hypothalamo-pituitary-thyroid (HPT) axis.
Moreover, a study of offspring of dams treated with OMC (500�1000 mg/kg/day) showed sex-dependent behavioral changes, namely decreased motor activity in females, but not in males, and improved spatial learning in males, suggesting that OMC can affect neuronal development, however the doses used in these experiments were extremely high, not relevant to possible human exposure.
Note: Study and data for tropical use onlyWarning: High dosage shall be avoided as reproductive system, thyroid and behavioral alterations in animal studies has been found, Tropical usage in breast area shall be avoided to prevent the OCTINOXATE passing orally in Infants.
Titanium dioxide and Breastfeeding
Low RiskNot much study has been done on effects of topical usage of Titanium Dioxide during breast feeding but as there is no finding of Titanium Dioxide passing in breast milk its unlikely to cause any health issue for infant.
Some animal studies suggest that maternal exposure to titanium dioxide nanoparticles during pregnancy and lactation alters offspring hippocampal mRNA BAX and Bcl-2 levels, induces apoptosis and decreases neurogenesis. But dosage was significantly higher than daily possible exposure to humans.
Note: Study and data for tropical use only. Inhalation concerns in powder or spray products.Warning: Tropical usage in breast area shall be avoided to prevent the Titanium Dioxide passing orally in Infants. Titanium dioxide is possibly carcinogenic to humans. Titanium dioxide can be drastically more harmful if used as powder or spray as risk of inhalation can increase significantly.
Octisalate and Breastfeeding
Low RiskOctyl salicylate is an oil soluble chemical sunscreen agent that absorbs UVB radiation. It does not protect against UVA. Octyl salicylate is used to augment the UVB protection in a sunscreen. Salicylates are weak UVB absorbers and they are generally used in combination with other UV filters
Octisalate rarely causes allergies in tropical usage. Not much study has been done on effects of topical usage of Octisalate during breast feeding however it is known to penetrate the skin hence it�s better to use other alternatives.
FDA study found blood levels 10 times above cutoff for systemic exposure, skin penetration in lab studies has been observed
Note: Study and data for tropical use onlyWarning: Tropical usage in breast area shall be avoided to prevent the Octisalate passing orally in Infants.
Oxybenzone and Breastfeeding
DangerousOxybenzone has been found in mother�s milk. And has relatively high 1% to 9% skin penetration in lab studies. Oxybenzone has relatively high rates of skin allergy, it has weak estrogen like effects, and its observed as moderate anti-androgen. Oxybenzone is associated with altered birth weight in human studies. It is not recommended to use Oxybenzone during breast feeding.
Note: Study and data for tropical use only.Warning: Tropical usage in breast area shall be avoided to prevent the Oxybenzone passing orally in Infants.
What should I do if already breastfed my kid after using Visible Difference Multi Targeted Bb Cream Broad Spectrum Sunscreen Spf 30 Shade 1?
You should immediately inform your health care provider about Visible Difference Multi Targeted Bb Cream Broad Spectrum Sunscreen Spf 30 Shade 1 usage and your breastfeeding interval after usage of
My doctor has prescribed me Visible Difference Multi Targeted Bb Cream Broad Spectrum Sunscreen Spf 30 Shade 1, what should I do?
Please double check with your doctor if he is aware of your breastfeeding stratus, Ask your doctor if there is any safe alternative of Visible Difference Multi Targeted Bb Cream Broad Spectrum Sunscreen Spf 30 Shade 1. Check with your doctor if you shall temporally stop breastfeeding. You may go for second opinion as well. Still after all of this if your doctor still recommends Visible Difference Multi Targeted Bb Cream Broad Spectrum Sunscreen Spf 30 Shade 1 then go for it as they have access on more detailed medical and scientific information and they understand your individual medical situation much better.
If I am using Visible Difference Multi Targeted Bb Cream Broad Spectrum Sunscreen Spf 30 Shade 1, will my baby need extra monitoring?
Extreme level of monitoring required as Visible Difference Multi Targeted Bb Cream Broad Spectrum Sunscreen Spf 30 Shade 1 could be dangerous for kid.
Who can I talk to if I have questions about usage of Visible Difference Multi Targeted Bb Cream Broad Spectrum Sunscreen Spf 30 Shade 1 in breastfeeding?
US
National Womens Health and Breastfeeding Helpline: 800-994-9662 (TDD 888-220-5446) 9 a.m. and 6 p.m. ET, Monday through Friday
UK
National Breastfeeding Helpline: 0300-100-0212 9.30am to 9.30pm, daily
Association of Breastfeeding Mothers: 0300-330-5453
La Leche League: 0345-120-2918
The Breastfeeding Network supporter line in Bengali and Sylheti: 0300-456-2421
National Childbirth Trust (NCT): 0300-330-0700
Australia
National Breastfeeding Helpline: 1800-686-268 24 hours a day, 7 days a week
Canada
Telehealth Ontario for breastfeeding: 1-866-797-0000 24 hours a day, 7 days a week
