Esika Pro Hidracolor 3 In 1 Moisturizing, Color And Perfecting Effect Spf 25 (pimienta Caliente) - Brown while Breastfeeding
What is Esika Pro Hidracolor 3 In 1 Moisturizing, Color And Perfecting Effect Spf 25 (pimienta Caliente) - Brown ?
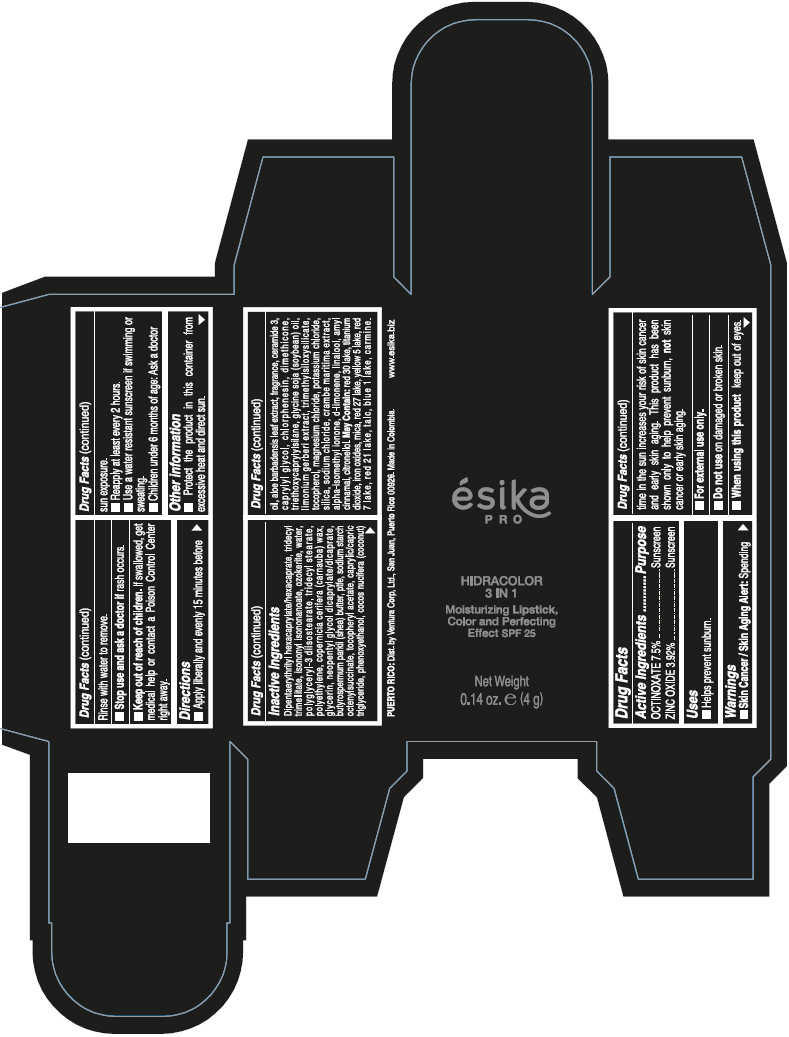
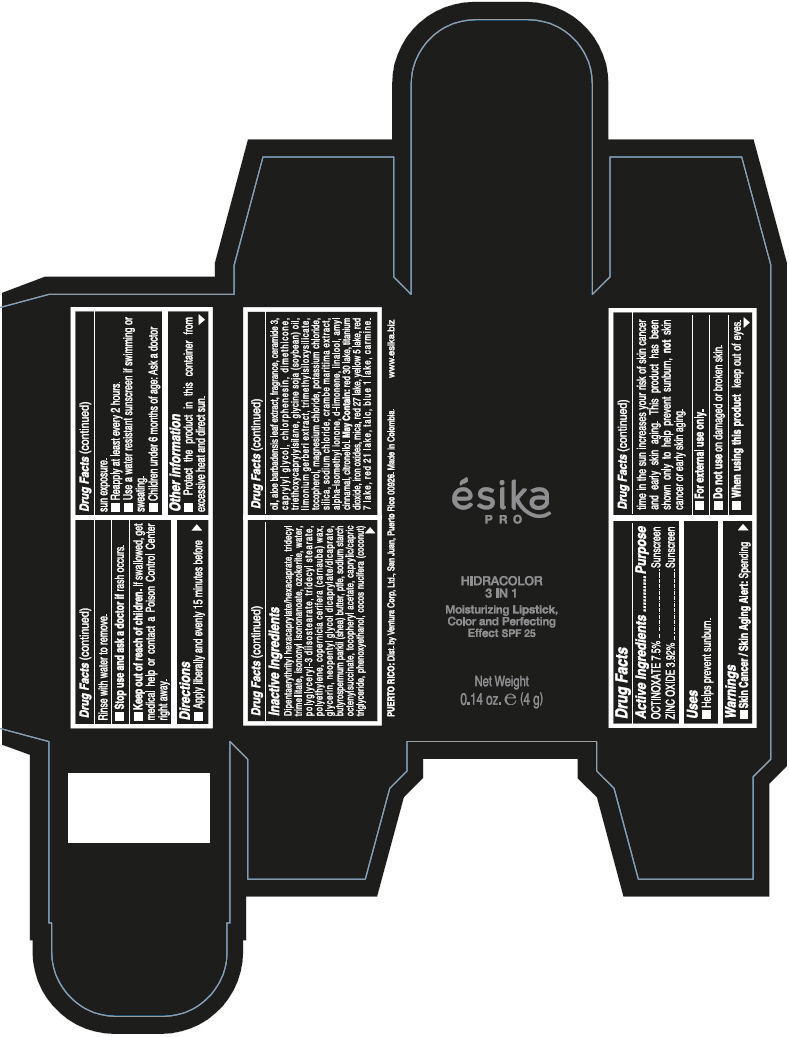
Brief: Active Ingredients Purpose OCTINOXATE 7.5% Sunscreen ZINC OXIDE 3.92% Sunscreen
Esika Pro Hidracolor 3 In 1 Moisturizing, Color And Perfecting Effect Spf 25 (pimienta Caliente) - Brown safe in breastfeeding?

Esika Pro Hidracolor 3 In 1 Moisturizing, Color And Perfecting Effect Spf 25 (pimienta Caliente) - Brown Breastfeeding Analsys
Zinc oxide while Breastfeeding
SafeCAS Number: 1314-13-2
It is used topically as an astringent and skin protector, very often together with small amounts of Ferric Oxide to form Calamine (see specific info). It is a product compatible with breastfeeding according to WHO Essential Medicine’s List - 2002.It is also used in dental hygiene products and cosmetics. Widely used for skin protection of the diaper area in infants. Because of the small dose used and poor absorption into plasma of most topical dermatological preparations, excretion into breastmilk in significant amount appears to be unlikely. Do not apply on the breast to prevent infant ingestion; otherwise, wash it off thoroughly with water before the next breast feed.
Esika Pro Hidracolor 3 In 1 Moisturizing, Color And Perfecting Effect Spf 25 (pimienta Caliente) - Brown Breastfeeding Analsys - 2
Octinoxate and Breastfeeding
Unsafe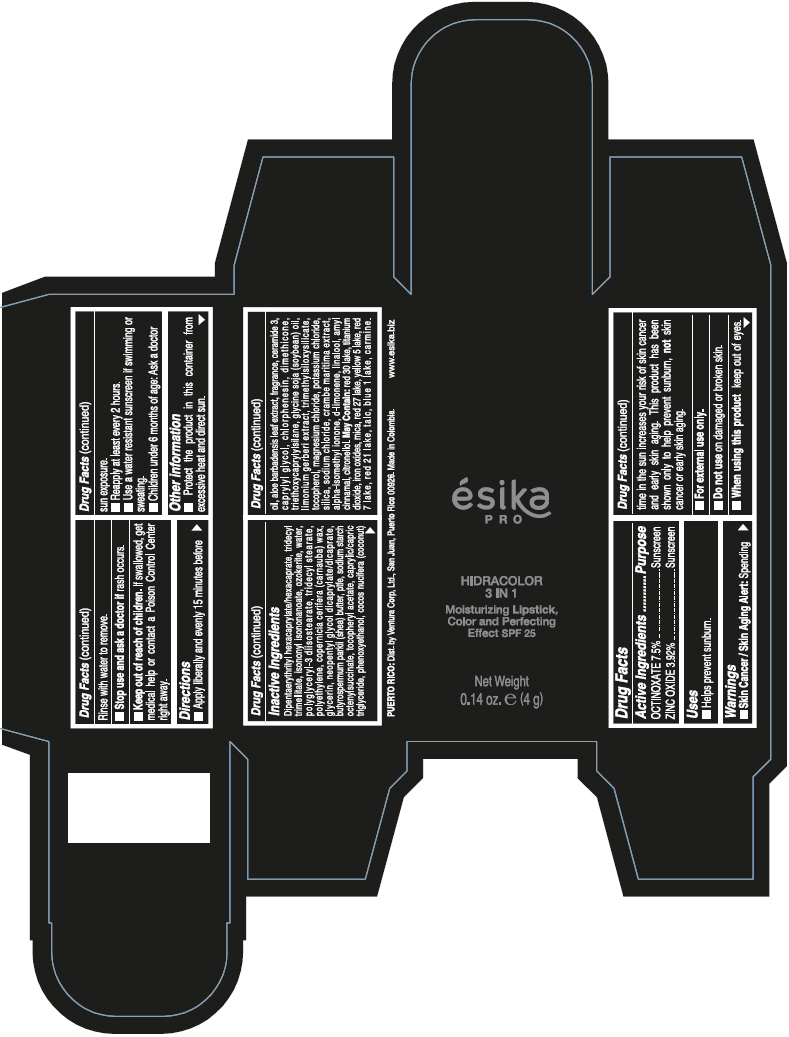
Octinoxate (Octylmethoxycinnamate) has been detected in human urine, blood and breast milk and is known for moderate risk of skin allergy. Some studies suggest that Octinoxate has estrogen like effects however less than 1% skin penetration has been found in human laboratory studies. As not much study has been done on effects of Octinoxate during breast feeding its recommended to use safe alternatives.
Octyl Methoxycinnamate (OMC) is a frequently used UV-filter in sunscreens and other cosmetics. Octinoxate can be systemically absorbed after skin application, being found in the deeper layers of the stratum corneum as well as urine, plasma, and breast milk. The mean maximum plasma concentration detected after application of 2mg/cm2 sunscreen was 7ng/mL in women and 16ng/mL in men. FDA study found blood levels 13 times above cutoff for systemic exposure.
Several studies indicated that OMC acts as an endocrine disruptor due to the ability to interfere with endocrine system at different levels. In humans OMC exposure has minor, but statistically significant effects on the levels of testosterone and estradiol. Moreover, some studies suggested that OMC can interact with the hypothalamo-pituitary-thyroid (HPT) axis.
Moreover, a study of offspring of dams treated with OMC (500�1000 mg/kg/day) showed sex-dependent behavioral changes, namely decreased motor activity in females, but not in males, and improved spatial learning in males, suggesting that OMC can affect neuronal development, however the doses used in these experiments were extremely high, not relevant to possible human exposure.
Note: Study and data for tropical use onlyWarning: High dosage shall be avoided as reproductive system, thyroid and behavioral alterations in animal studies has been found, Tropical usage in breast area shall be avoided to prevent the OCTINOXATE passing orally in Infants.
What should I do if already breastfed my kid after using Esika Pro Hidracolor 3 In 1 Moisturizing, Color And Perfecting Effect Spf 25 (pimienta Caliente) - Brown?
We have already established that Esika Pro Hidracolor 3 In 1 Moisturizing, Color And Perfecting Effect Spf 25 (pimienta Caliente) - Brown is unsafe in breastfeeding and breastfeeding while using Esika Pro Hidracolor 3 In 1 Moisturizing, Color And Perfecting Effect Spf 25 (pimienta Caliente) - Brown is not a good idea however if have already used
My doctor has prescribed me Esika Pro Hidracolor 3 In 1 Moisturizing, Color And Perfecting Effect Spf 25 (pimienta Caliente) - Brown, what should I do?
If your doctor knows that you are breastfeeding mother and still prescribes Esika Pro Hidracolor 3 In 1 Moisturizing, Color And Perfecting Effect Spf 25 (pimienta Caliente) - Brown then there must be good reason for that as Esika Pro Hidracolor 3 In 1 Moisturizing, Color And Perfecting Effect Spf 25 (pimienta Caliente) - Brown is considered unsafe, It usually happens when doctor finds that overall advantage of taking
If I am using Esika Pro Hidracolor 3 In 1 Moisturizing, Color And Perfecting Effect Spf 25 (pimienta Caliente) - Brown, will my baby need extra monitoring?
Yes, Extra monitoring is required if mother is using Esika Pro Hidracolor 3 In 1 Moisturizing, Color And Perfecting Effect Spf 25 (pimienta Caliente) - Brown and breastfeeding as it is considered unsafe for baby.
Who can I talk to if I have questions about usage of Esika Pro Hidracolor 3 In 1 Moisturizing, Color And Perfecting Effect Spf 25 (pimienta Caliente) - Brown in breastfeeding?
US
National Womens Health and Breastfeeding Helpline: 800-994-9662 (TDD 888-220-5446) 9 a.m. and 6 p.m. ET, Monday through Friday
UK
National Breastfeeding Helpline: 0300-100-0212 9.30am to 9.30pm, daily
Association of Breastfeeding Mothers: 0300-330-5453
La Leche League: 0345-120-2918
The Breastfeeding Network supporter line in Bengali and Sylheti: 0300-456-2421
National Childbirth Trust (NCT): 0300-330-0700
Australia
National Breastfeeding Helpline: 1800-686-268 24 hours a day, 7 days a week
Canada
Telehealth Ontario for breastfeeding: 1-866-797-0000 24 hours a day, 7 days a week
